 .
. .
.
barleypopmaker wrote:but I'm thick skulled when it comes to learning extremly dry science materials.

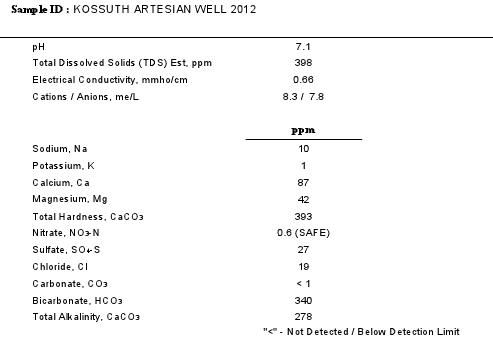
mabrungard wrote:That water is quite hard, but more importantly is that it is very alkaline. Part of the hardness is an excessive concentration of magnesium. This water is well suited for lime softening to help reduce alkalinity and hardness. An Excess Lime process is necessary for reducing the magnesium content. Alternatively, RO water may be an easier route.
Its not a total surprise that PA, IPA, and stout come out better with this water. But they can be even better with a less mineralized water source. Diluting the source water with RO at a rate of at least 1 to 1 is needed to bring the Mg and sulfate to more modest levels. The alkalinity will also be cut in half, but acidification may be needed for some brews to allow the mash pH to drop into the proper range.
Do visit the Bru'n Water website and read the Water Knowledge page to get a better understanding of what that water report tells you and why you should consider treatment.
brewindruid wrote:What happens to the ph when you dilute?
Another question is I have a supplement that is a calcium magnesium supplement, but a percentage of the supplement is Calcium citrate, can I still use it?


Users browsing this forum: No registered users